Assessment of Transcutaneous Electrical Nerve Stimulation Efficacy in Pain Management and Muscle Recovery: A Controlled Clinical Trial for Exercise-Induced Muscle Fatigue.
Adriana M Degani, PT MSc PhD1; Hannah Stefanek, DPT2; Josh Sparks, DPT2; Patrícia Driusso PT MSc PhD 3; Alessander Danna-dos-Santos PT MSc PhD 1,2, Daryl Lawson, PT DSc 1,2
1. Laboratory for Advancements in Rehabilitation Sciences (LARS), Department of Physical Therapy, Western Michigan University, Kalamazoo, MI, USA
2. Women’s Health Research Laboratory, Department of Physical Therapy, Federal University of São Carlos, São Carlos, SP, Brazil
3. Department of Physical Therapy, Western Michigan University, Kalamazoo, MI, USA
Purpose: To investigate the efficacy of transcutaneous electrical nerve stimulation (TENS) on the duration and magnitude of muscle pain and fatigue following an exercise-induced fatigue pro-tocol.
Methods: Twenty-one participants were tested over three visits. Each visit consisted of an exercise-induced fatigue protocol to the quadriceps muscle followed by one of the three randomized independent treatments: no intervention (NoTENS), TENS on the fatigued quadriceps (TENSactive), and sham intervention with an inactive TENS (TENSinactive) on the fatigued quadriceps. The visual analog scale (VAS) for perceived muscle pain and fatigue levels was recorded before and after exercise, immediately after the designated follow-up protocol, and 24 and 48 hours after the visit.
Results: Post-exercise muscle pain and fatigue decreased immediately following active TENS application to the quadriceps; this decrease persisted 24 hours post-TENS application. Muscle pain and fatigue immediately after active TENS were significantly lower than NoTENS and TENSinactive treatments. The active TENS also showed significantly faster recovery compared to NoTENS and TENSinactive protocols.
Conclusions: TENS application immediately after muscle fatigue following exercise was more effective in decreasing muscle pain and muscle fatigue, and improving muscle recovery time compared to no TENS or sham TENS. These findings support the use of TENS as a non-invasive, non-pharmacological evidence-based protocol to manage muscle pain and fatigue after exercise, training, and sports, resulting in reduced muscle pain and fatigue, and shortened muscle recovery time after strenuous exercise for athletes and non-athletes aged between 18 and 45 years old.
Skeletal muscle fatigue is defined as a decrease in the efficiency of muscle force production in response to comparable levels of contractile activity. The most common development mechanisms for muscle fatigue are associated with both metabolic responses to exercise and inflammatory responses elicited by the micro-structural dam-ages imposed on the muscle cells and their connective strata.1,2 As a result, a broad range of symptoms might emerge, such as varying levels of pain severity, decreased muscle strength and power, functional impairment, changes in joint mechanics, and changes in muscle recruitment patterns.2,3 Multiple interventional protocols aiming to mitigate these fatiguing symptoms have been proposed, including anti-inflammatory medication, cryotherapy, muscle stretching, nutritional supplementation, massage therapy, hyperbaric oxygen therapy, ultrasound therapy, and electrical stimulation techniques.2-4
Transcutaneous electrical nerve stimulation (TENS) is a non-pharmacological intervention commonly used in clinical settings to manage pain symptoms and facilitate return to activity. TENS uses an electrical current through electrodes placed on the skin to stimulate the sensory and motor nerves for therapeutic purposes, such as pain con-trol.4 TENS preference as an interventional method is rooted in its non-invasive nature, relatively easy application, and safety. However, TENS effectiveness in addressing acute muscle pain and fatigue remains uncertain. To understand TENS significance, it is necessary to investigate the theoretical foundation and mechanisms of TENS. The role of TENS on modulating muscle pain and fatigue sensation is based on the potential effects of TENS in the sensory modulation of pain pathways, the release of endogenous opioids, and the improvement of blood flow and metabolism5-13. TENS can modulate pain pathways in the nervous system. Central mechanisms, operating in the spinal cord and brainstem, release opioids and neurotransmitters, aiding pain relief and decreasing the sensation of muscle fatigue.5,6 Peripheral mechanisms seem to activate alpha and beta large-diameter afferent fibers, inhibiting nociceptive neurons.5-9 TENS can also improve local blood flow and metabolism in fatigued muscles, which may help alleviate muscle pain, enhance muscle recovery, and reduce the sensation of fatigue.11
However, the controversial efficacy of TENS in fatigue-related pain management and inconsistent findings are reported across the current literature. For example, while a few studies suggest significant reductions in perceived pain when TENS is used during exercise or up to 48 hours following delayed muscle onset soreness,14-16 others report no effects on pain management when used prior, during, or up to 72 hours after the execution of strenuous exercise.17-19 This lack of agreement in the literature on the analgesic effects of TENS on post-exercise muscle pain may be related to the variability in TENS protocols used for pain management.
Based on the promising effects of TENS on acute pain management, the present study investigated whether the immediate application of TENS following exercise-induced muscle fatigue, compared to no intervention, would result in a reduced duration and level of muscle pain and fatigue, as well as improved muscle recovery time. We hypothesized that TENS applied immediately after an exercise-induced fatigue protocol will better reduce muscle pain and fatigue while improving muscle recovery time compared to sham or no intervention.
2. Materials and Methods
Participants. Participants were recruited through advertisements strategically placed within the university campus and fitness centers, utilizing both physical media such as flyers and posters, as well as digital platforms to maximize visibility. The advertisements not only served to inform potential participants directly but also encouraged word-of-mouth referrals, leveraging interpersonal networks to further amplify our recruitment efforts. Inclusion criteria for participation consisted of healthy adults of any gender aged between 18 and 45 years old who agreed to refrain from using pain or an-ti-inflammatory medication throughout the entire study duration. Exclusion criteria consisted of diagnosed preconditions including diabetes mellitus, neuropathy, dementia, uncontrolled hypertension, neuromuscular disorders, or any major joint pain (e.g., back, hip, or knee) that could limit functional ability. In addition, individuals who have previously used TENS or for whom TENS is contraindicated were excluded from this study. Contraindications may include implanted pacemakers, dermatological conditions in the area of TENS treatment, abnormal sensation in the lower extremities, or allergy to the saline media and adhesive materials present on the surface of the electrodes.
Ethical Considerations. This study follows ethical guidelines. This study was ap-proved by the local Institutional Review Board and was in conformity with The Declaration of Helsinki. All participants voluntarily provided their informed consent before participating in this study.
Materials
In contrast, the inactive TENS unit was programmed to deliver no current, serving as a sham protocol. However, its appearance, operation, display, and instruction closely mirrored those of the active unit to prevent participant bias. Participants were informed about the sensations they might experience during stimulation and were encouraged to communicate any discomfort or pain immediately. Participants were also informed that they may not feel any sensations. This can occur when the electrical current is set at a low intensity, which may not be perceptible to everyone. This explanation provided a plausible reason for the lack of sensation when using the inactive TENS without explicitly stating that this is a sham TENS unit.
- Isokinetic dynamometer (Cybex 6000, Cybex International, Inc.): An isokinetic dynamometer (Figure 1A) was used to induce muscle fatigue in the right quadriceps and to record the muscle maximal efficiency, expressed in muscle peak torque (MPT). Mus-cle fatigue is characterized by a decrease in maximal force or power production due to repeated or sustained contractile activity. The isokinetic dynamometer is widely recognized for its ability to induce muscle fatigue, with several studies successfully employing flexion/extension exercise protocols to decrease peak torque and induce quadriceps fatigue.7-9,21 Despite variations in the number of sets, repetitions, and angular velocity of knee flexion/extension among these studies, all reported a significant re-duction in the quadriceps peak torque.7-9,21
- TENS units (HV-F080, Omron Healthcare, Inc.): Active and inactive TENS units were used in this study. Before electrode application, the skin was cleansed with isopropyl alcohol. Electrodes, self-adhesive with dimensions of 5 cm by 5 cm, were positioned on the anterior surface of the thigh, precisely over the quadriceps muscle, at a location corresponding to the midpoint of the muscle belly (Figure 1B). The placement followed a longitudinal alignment with the muscle fibers. To ensure consistency be-tween active and inactive trials, electrode placement was replicated whether using an active or inactive TENS.
The TENS unit was configured in "recovery mode," a sensory-level setting in TENS model HV-F080 (Omron Healthcare, Inc .) designed to facilitate muscle relaxation, reduce fatigue, and enhance muscle recovery. Stimulation parameters included a biphasic pulse with a duration of 100 µsec and a frequency modulated between 0.2 Hz and 99 Hz. Notably, frequency modulation was employed to prevent nerve adaptation, thereby maintaining responsiveness to the stimulation. The intensity of the electrical current was participant-controlled, with a maximum limit of 50 Volts. Participants were instructed to gradually increase the intensity until reaching a comfortable sensory level characterized by a tingling or buzzing sensation, without discomfort or pain. This method ensured individualized and optimized sensory stimulation.
Throughout the stimulation period, which lasted for 30 minutes, the current was delivered in discrete pulses, indicating that the electrical impulses were delivered in separate, distinct bursts rather than continuously. The ratio of time the electrical stimulation was active (on) to the time it was inactive (off) during each cycle of stimulation, known as the on/off time or duty cycle, was preset by the manufacturer. This intermittent delivery method aimed to enhance efficacy and mitigate potential adverse effects associated with prolonged continuous stimulation. - Visual Analog Scale (VAS): The VAS was used to assess muscle pain (VASpain) and muscle fatigue (VASfatigue) of the quadriceps in several stages of this study. The VAS is a widely used psychometric instrument commonly employed to measure subjective or self-reported experiences, particularly those related to pain, discomfort, or other sensations.21-23 Participants were instructed to indicate the place on the line cor-responding to their muscle pain and fatigue self-perceived state utilizing one continuous sliding scale of 0-100 mm to measure quadriceps pain and another similar scale to measure quadriceps fatigue. The lower and upper ends of the scale represented no muscle pain or fatigue and the most extreme muscle pain or fatigue, respectively.
- The Borg Rating Perceived Exertion (RPE): RPE was used to measure the perception of exertion after exercising on a scale from zero (“no exertion”) to ten (“maximal exertion”).24
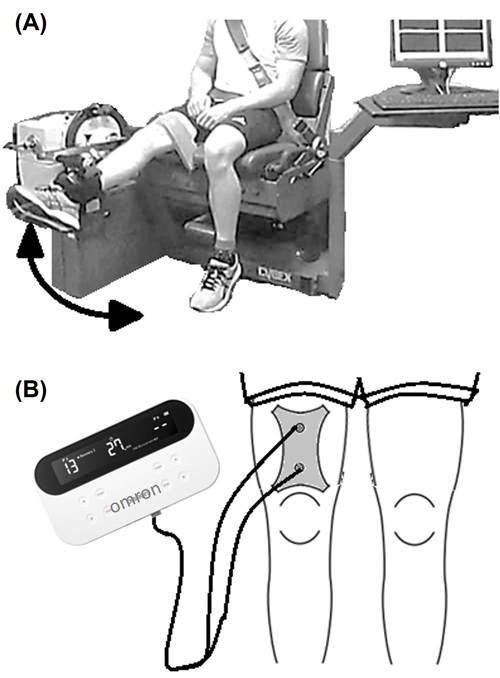
Figure 1. Illustrative image of (A) a knee isokinetic flexion and extension using the isokinetic dynamometer, and (B) a participant using the TENS unit after exercising.
Experimental Design. A controlled clinical trial with a crossover design with repeated measurements and a washout period of 10 days was used to avoid any potential carryover effects.
Experimental Procedure. This study was organized into three phases: baseline, TENS intervention, and sham intervention. The order of intervention was randomly assigned. Each phase has one visit and 10-day follow-up measurements. The first phase consisted of baseline metrics for the natural course of muscle fatigue recovery. In the second and third phases, interventional and sham protocols were introduced. The experimental protocol is outlined as follows:
- Baseline recordings (days 1 to 11): The first visit began with using the isokinetic dynamometer to measure the baseline quadriceps muscle peak torque (MPTbaseline), which was considered the maximal functional capacity of the quadriceps muscle to execute knee extension at 60o/sec. The MPTbaseline was obtained as the maximum MPT value out of five repetitions, measured in newtons (N). This value was used to establish the fatiguing threshold of each participant. In sequence, each participant was submitted to an exercise-induced fatigue (EIF) protocol composed of blocks of full isokinetic knee flexion/extension actions performed at 180o/s, 120o/s, and 60o/s. Each block consisted of three sets of ten repetitions. These blocks were repeated until the maximum peak torque delivered by the quadriceps at 60o/s declined to a threshold corresponding to 65% of the participants' MPTbaseline. Once this threshold was reached, the quadriceps muscle was considered fatigued, and the average peak torque recorded across the last 60o/s repetitions was obtained (MPTfinal).
The protocol implemented to induce muscle fatigue was based on current EIF protocols for the quadriceps muscle,25 and the use of the percentage of MPT reduction to characterize muscle fatigue minimizes any potential effects of gender, muscle size, and strength that could influence the outcomes of this study.
Immediately after the EIF protocol, the participant was asked to self-report their level of exertion using the Borg Rating Perceived Exertion (RPE) and their level of muscle pain and fatigue using the visual analog scale (VASpain and VASfatigue, respectively). After 30 minutes of resting, participants were asked to self-report VASpain and VASfatigue again.
Participants were instructed to record VASpain and VASfatigue at home once a day during the ten days following the fatiguing protocol to record the natural course of fatigue recovery.
- Interventional and sham protocols (days 12 to 33): Following the natural recovery of the quadriceps muscle, all participants were submitted to an interventional protocol using an active TENS (TENSactive) and a sham protocol using an inactive TENS (TENSinactive). The order of the protocols was randomly assigned for each participant. One protocol was performed on days 12 to 22, and the other protocol was performed on days 23 to 33. All procedures and measurements performed between days 1 to 11 of this study were repeated for both protocols. However, a TENS session was administered for 30 minutes instead of the 30 minutes of resting performed on day 1 of the baseline phase.
Data Analysis and Statistical Approach. Statistical tests were performed using the IBM SPSS statistics software (version 22). The primary response variables included the muscle pain and fatigue levels recorded using the VAS at different time periods (before and immediately after the EIF protocol, 30 minutes after exercising with and without using TENS, and in the following days after exercising), changes in VAS, muscle pain and fatigue recovery time, percentage of individuals reporting no muscle pain or fatigue at different moments, and percentage of clinically important muscle pain or fatigue reduction at different moments, which was calculated using Relative Change Index (RCI) computations.26 Secondary response variables included the maximal voluntary quadriceps contraction before and after the EIF protocol, the percentage of baseline peak torque during the EIF protocol, and the rating of perceived exertion immediately after the EIF protocol.
The normality assumption was checked using Shapiro-Wilk tests. Since some variables did not follow a normal distribution, non-parametric Friedman and Wilcoxon Signed Rank tests were used to investigate the effects of TENS (NoTENS, TENSinactive, and TENSactive) on the primary outcomes. For all response outcomes, the median, first quartile, and third quartile across all 21 participants were reported. The significant level was fixed at 0.05.
3. Results
A total of twenty-one eligible individuals (16 females/5 males, mean age of 23.2 ± 4.0 years old, mean weight of 78.3 ± 22.3 kg, and mean BMI of 26.9 ± 6.6 kg/m2 participated in this study. Kruskal-Wallis tests revealed no significant effect of gender on either age or BMI (p < 0.05).
The EIF implemented in this study decreased the quadriceps muscle peak torque (MPT) from 105 N (91, 128) to 58 N (51, 75) (median and quartile values). The resulting MPT decreased to 54.7% (51.7, 60.1), indicating successful elicitation of fatigue in participants’ quadriceps muscles. Additionally, the exercise led to high perceived exertion levels (median RPE 7.0, quartiles 6.5, 8.0), ranging from 5 (difficult) to 9.5 (extremely hard), and clinically significant increase in muscle pain (median VASPain 3.5, quartiles 2.5, 6.0) and fatigue (median VASFatigue 6.0, quartiles 4.0, 8.0).
Table 1 and Figures 2 and 3 show the self-reported muscle pain and fatigue levels at different time points after the fatigue-inducing protocol. Friedman's tests revealed a significant effect of the interventional protocol (NoTENS, TENSinactive, and TENSactive) on VAS for both muscle pain and muscle fatigue levels (see Friedman's values in Table 1). Post-hoc analysis with Wilcoxon signed-rank tests revealed significantly lower muscle pain 30 minutes after exercising when the active TENS was used compared to no TENS or inactive TENS (p = 0.002 and p = 0.010, respectively), and 48 hours after exercising (p = 0.050 and p = 0.012, respectively). Perceived muscle fatigue was also significantly lower 30 minutes after exercising when the active TENS was used compared to no TENS or inactive TENS (p < 0.001 and p < 0.001, respectively), 24 hours after exercising (p = 0.011 and p = 0.013, respectively), and 48 hours after exercising (p < 0.001 and p = 0.001, respectively).
Muscle pain and fatigue levels decreased 30 minutes and 24 hours after intervention (with or without TENS application) are presented in Table 1 and Figure 3. The active TENS was clinically meaningful in reducing muscle pain and fatigue immediately after intervention to exhaustion based on RCI values. The series of Friedman's tests showed a significant effect of the interventional protocol on the changes in both VAS pain and fatigue 30 minutes and 24 hours after the EIF protocol. Post-hoc analysis with Wilcoxon signed-rank tests revealed significantly larger decreases in muscle pain and fatigue after TENSactive application 30 minutes (p < 0.001 and p < 0.001, respectively) and 24 hours after intervention (p = 0.018 and p = 0.022, respectively) compared to Baseline. Muscle pain and fatigue decreases after using the TENSactive were also significantly higher 30 minutes after intervention compared to the TENSinactive (p = 0.010 and p <0.001, respectively). In addition, muscle fatigue decreased significantly 24 hours after intervention and using the active TENS compared to the inactive TENS (p = 0.013). Muscle pain reduction using the inactive TENS was greater when compared to not using TENS after intervention and 24 hours after intervention (p = 0.021 and p = 0.004, respectively).
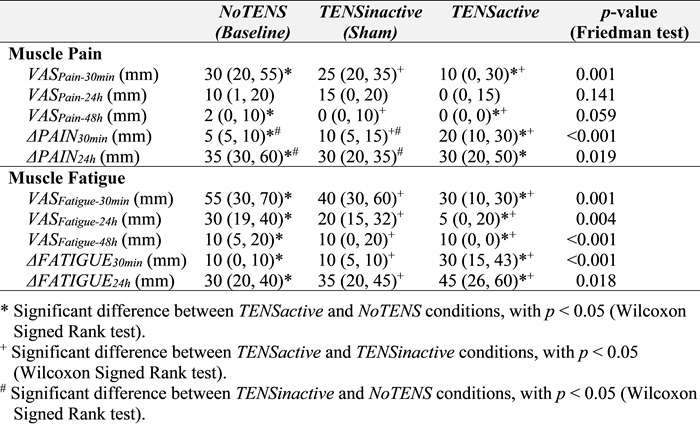
Table 1. Median and quartiles for the muscle pain and fatigue levels (VASPain and VASFatigue, respectively) at different time points, and the decrease in muscle pain and fatigue (ΔPAIN and ΔFATIGUE, respectively) after the fatigue-inducing protocol
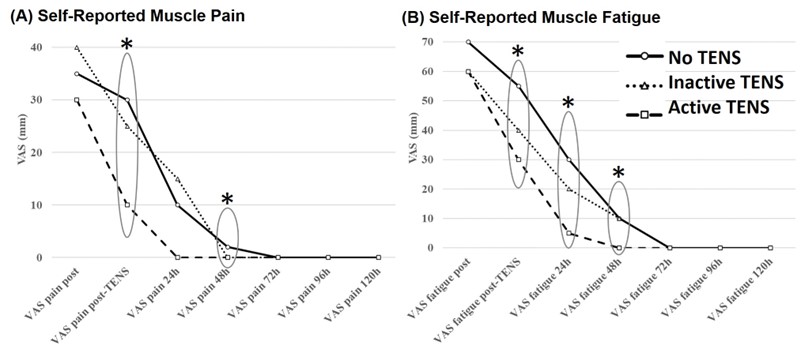
Figure 2. The median across participants of self-reported muscle (A) pain and (B) fatigue levels over time. Note: * Significant difference between with p < 0.05 (Friedman tests).
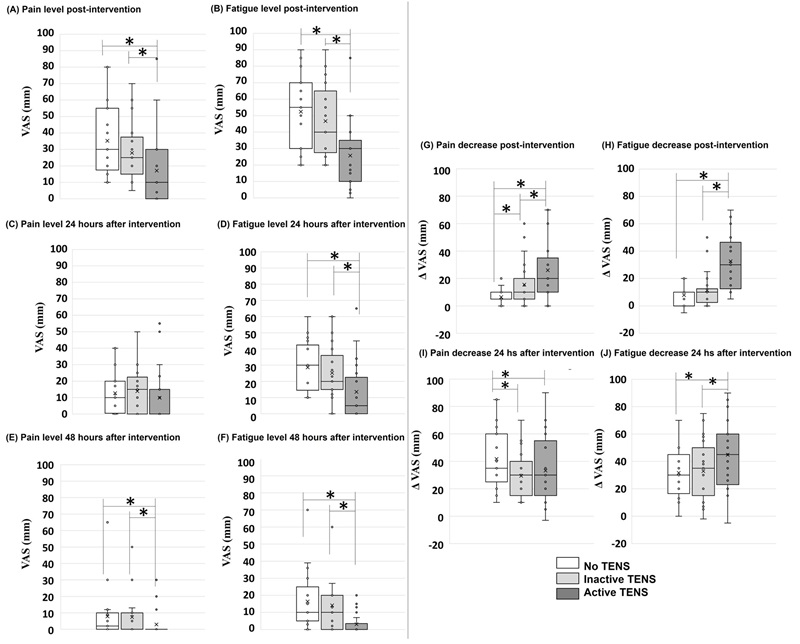
Figure 3. Box plots of self-reported muscle pain and fatigue levels (A and B) immediately after intervention, (C and D) 24 hours after intervention, and (E and F) 48 hours after intervention. Boxplots of the muscle (G and I) pain and (H and J) fatigue decrease 30 minutes and 24 hours after intervention (with or without TENS application). Note: * Significant difference with p < 0.05 (Wilcoxon Signed Rank tests).
Table 2 and Figure 4 show the time (in days) when VASPain and VASFatigue fully resolved and reached a score of zero. Friedman's tests showed a significant effect of the interventional protocol on the duration of both muscle pain and fatigue after the EIF protocol. Post-hoc analysis with Wilcoxon signed-rank tests revealed a significant de-crease in muscle pain and fatigue duration for TENSactive when compared to both NoTENS (p < 0.001 and p < 0.001, respectively) and TENSinactive (p < 0.001 and p < 0.001, respectively).
The percentage of reports indicating complete regression of symptoms (muscle pain and fatigue) at different time points after the fatigue-inducing protocol for each interventional protocol is presented in Table 2 and Figure 4CD. No self-reported muscle pain or fatigue 30 minutes after exercising was reported only when the active TENS was applied. Muscle fatigue was also absent after 24 hours in 47.6% of the participants when the active TENS was applied after exercising. No participants reported zero fatigue the next day when no TENS was applied after exercise. On the second day, higher percentages of participants reporting no muscle pain or fatigue were found when the active TENS was used immediately after exercise compared to no TENS (Table 2).
The percentage of clinically significant muscle pain and fatigue decrease 30 minutes and 24 hours after exercise for all three interventional protocols are reported in Table 2 and Figure 4EF. Note the higher percentage of clinically significant muscle pain or fatigue decreases immediately after the active TENS treatment compared to no TENS treatment.
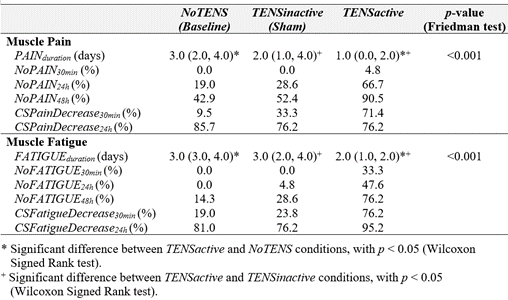
Table 2. Median and quartiles for the subsidence time (PAINduration and FATIGUE-duration) and symptom regression (NoPAIN and NoFATIGUE) at different time points, and the clinically significant drop (CSPainDecrease and CSFatigueDecrease) after exercise for all interventions (NoTENS, TENSinactive, and TENSactive)
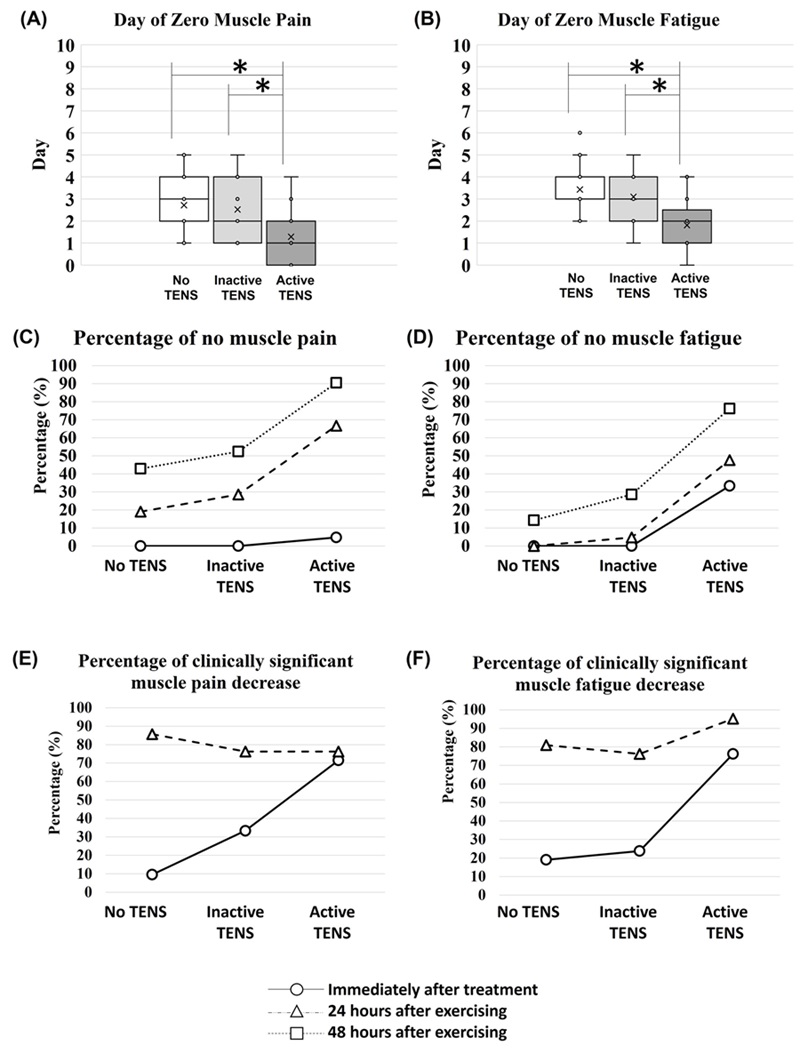
Figure 4. Boxplots of the day that muscle (A) pain and (B) fatigue levels were reported as zero. The percentage of no muscle (C) pain and (D) fatigue immediately after treatment, 24 and 48 hours after exercising, and the percentage of clinically significant muscle (E) pain and (F) fatigue decrease immediately after treatment and 24 hours after exercising. Note: * Significant difference with p < 0.05 (Wilcoxon Signed Rank tests).
4. Discussion
Muscle pain, soreness, and fatigue after strenuous and/or prolonged exercise are common symptoms during sports, training, and/or leisure physical activities. These symptoms are also frequent complaints in clinical practice and might affect athletic performance and daily life. Therefore, we investigated the effects of TENS applied immediately after an EIF protocol on the duration and level of muscle pain and fatigue.
Despite the widespread utilization of TENS to alleviate acute muscle pain, differences in population and painful conditions, sample size, TENS parameters, and study designs have also challenged the evidence for the use of TENS in acute muscle pain. The possibility of participants employing additional pain management strategies during these studies could potentially lead to disparate outcomes. In the current study, compliance with the restriction on using pain and anti-inflammatory medication was monitored to prevent any misinterpretation of the outcomes. Studies support that effective pain management depends on appropriate treatment protocols.27,28 In general, the pre-sent study supports using TENS with sensory-level stimulation immediately after strenuous exercise to manage acute muscle pain and fatigue and decrease recovery time. Lower muscle pain and fatigue levels immediately after TENS application and in the following days were reported, compared to no TENS treatment or inactive TENS. Positive findings in the literature also support the use of TENS on acute muscle pain. For example, studies on DOMS showed muscle pain reduction when TENS was applied 24 hours and/or 48 hours after exercising.29 TENS also reduced pain summation during exercise and increased physical tolerance when used during exercise 24 hours after a DOMS-induced exercise protocol.30 In another study, lower muscle pain after strenuous isometric exercise of the biceps brachii muscle was reported when the TENS was used during the exercise.14 The present study used a different protocol. TENS was applied immediately after strenuous exercise instead of waiting 24 hours to start treating muscle pain and fatigue. Despite not being related to acute muscle pain, current literature shows positive effects of TENS in the management of chronic pain and other disorders.4,20,31-33 In addition, a significant decrease in the use of pain medication and physical therapy was reported in the long-term use of TENS to manage chronic pain, resulting in a reduction of medication-related complications and pain treatment costs.34
A few studies reported no evidence for the use of TENS on exercise-induced muscle pain. The main differences between these studies and the present one are the TENS protocol applied and the pain-related variables used to evaluate the efficacy of the treatment. A TENS protocol before strenuous bicycling exercise found no significant improvement in performance or difference in pain level during exercise.18,19,36 A TENS protocol during strenuous exercise revealed similar pain level reports compared to placebo TENS.19 However, there was no control group. Other studies applied TENS 24, 48, and/or 72 hours after exercise-induced DOMS. These studies found no significant differences in pain levels compared to control groups.17,35 In a study using TENS im-mediately after strenuous exercise, no effects of TENS on either muscle fatigue index or fatigue markers in the blood were found,36 however, the level of muscle pain was not investigated. In our study, TENS was applied immediately after an isokinetic EIF in-tended to induce muscle fatigue, with a focus on muscle pain and fatigue recovery. A series of variables was computed based on self-reported muscle pain and fatigue. Muscle pain and fatigue reductions immediately after TENS application were higher than in the control group. It was also clinically meaningful based on RCI computations performed in this study.
As important as muscle pain and fatigue management are after strenuous exercise, recovery time is crucial to resume regular daily and/or sports activities. Most of the current studies explored the use of TENS only when DOMS symptoms oc-curred.16,17,29,35,37 Our results provided evidence for the analgesic effects of TENS upon muscle pain and fatigue when used immediately after strenuous exercise and at the stimulation parameters used here. Significantly faster muscle pain and fatigue recovery time were reported after exercise when TENS was applied. No muscle pain was re-ported in 4.8% of the participants and no muscle fatigue was reported in 33.3% of the participants immediately after using TENS. When TENS was not used after exercising, this finding was absent. The percentage of participants with no muscle pain and fatigue was also higher in the next 24 and 48 hours when TENS was applied immediately after exercising. Since muscle pain can be present for hours after strenuous exercise, fol-low-ups in self-reported muscle pain and fatigue levels should be added in future studies to understand better the effects of TENS on pain management after exer-cise-induced muscle fatigue.
Although the effects of a placebo treatment were not this study's goal, some findings are worth discussing. The use of TENS leads to lower levels of muscle pain and fatigue, and higher and clinically meaningful muscle pain and fatigue decreases com-pared to the inactive TENS treatment. Muscle pain and fatigue recovery were also faster when using the active TENS. While there was no significant difference in muscle pain and fatigue levels between the sham protocol (inactive TENS) and the baseline (no TENS), it's noteworthy that there was a significantly higher decrease in muscle pain and fatigue immediately after the inactive TENS compared to 30 minutes after no TENS intervention. Additionally, the proportion of participants experiencing a clinically meaningful reduction in muscle pain after inactive TENS was notably higher compared to the protocol with no TENS application (33.3% and 9.5%, respectively for inactive TENS and no TENS treatments).
Despite not being the aim of this study, the EIF protocol implemented was suc-cessful in achieving quadriceps fatigue. The quadriceps peak torque decreased to about 54.7% of its baseline. In addition, muscle pain and fatigue increase after exercising were clinically meaningful and still present 24 and 48 hours after exercising, followed by no treatment. This finding reinforces the delayed-onset muscle soreness (DOMS) effect of the exercise protocol used in this study.
Several important limitations and challenges are present in this study. The small sample size and potential engagement of participants in physical activities may limit the applicability of the findings to a broader population encompassing diverse age groups and activity levels. Despite efforts to standardize the application of TENS intervention, its effectiveness might be influenced by individual variations in pain tolerance and perception. Additionally, focusing exclusively on the quadriceps muscle warrants cau-tion when extrapolating these findings to other muscle groups. Furthermore, the ab-sence of direct measurement of adipose tissue at the stimulation site, which can affect electrical stimulation administration (e.g., TENS), is noteworthy and could potentially impact the results. Lastly, participant recruitment and compliance posed challenges, particularly given that this study was conducted during the COVID-19 pandemic.
5. Conclusion
This study presents an evidence-based protocol for managing acute muscle pain resulting from exercise. Clinically significant reduction in muscle pain and fatigue oc-curred immediately following TENS application to the quadriceps muscle. These effects persisted for several days. Moreover, muscle pain and fatigue symptoms dissipated earlier. The TENS protocol applied in this study was shown to be effective as a non-invasive, non-pharmacological evidence-based protocol to be applied immediately after exercise-induced fatigue, resulting in reduced muscle pain and fatigue, and shortened muscle recovery time. In addition, while an expedited return to physical ac-tivity and sports was not measured in this study and no conclusion can be drawn, it may be a plausible outcome. Further research is warranted to substantiate this potential benefit.
Therefore, findings from this study hold clinical importance for both athletes and non-athletes who experience acute muscle pain and delayed-onset muscle soreness due to exercise, ultimately reducing the delays in muscle recovery from fatigue.
Acknowledgments
The authors would like to acknowledge Sailesh Satpathy (BS, PT, CEAS, Cert. MDT, CMP, CIDN) and Auro Physical Therapy for providing the physical space and granting the use of their isokinetic dynamometer for this study. The authors would like to acknowledge Omron Healthcare, Inc. for providing active and inactive TENS devices for this study. References
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 2001; 81: 1725–1789. doi: 10.1152/physrev.2001.81.4.1725.
- Wan J-J, Qin Z, Wang P-Y, et al. Muscle fatigue: general understanding and treatment. Experimental & Molecular Medicine 2017; 49(10): e384. doi: 10.1038/emm.2017.194.
- Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 2003; 33(2): 145-164. doi: 10.2165/00007256-200333020-00005.
- Vance CGT, Dailey DL, Rakel BA, et al. Using TENS for pain control: the state of the evidence. Pain Management 2014; 4(3): 197-209. doi: 10.2217/pmt.14.13.
- Sabino GS, Santos CM, Francischi JN, et al. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain 2008; 9: 157–163. doi:10.1016/j.jpain.2007.09.003.
- Sluka KA. The Neurobiology of pain and foundations for electrical stimulation. In: Robinson AJ, Snyder-Mackler L, ed. Clinical Electrophysiology. Philadelphia: Lippincott Williams & Wilkins; 2008: 107-149.
- Hirano M, Katoh M, Gomi M, et al. Validity and reliability of isometric knee extension muscle strength meas-urements using a belt-stabilized hand-held dynamometer: a comparison with the measurement using an iso-kinetic dynamometer in a sitting posture. J Phys Ther Sci 2020; 32(2): 120-124. doi:10.1589/jpts.32.120.
- Johnson M. Transcutaneous electrical nerve stimulation: mechanisms, clinical application and evidence. Rev Pain 2007; 1(1): 7-11. doi: 10.1177/204946370700100103.
- Johnson MI, Paley CA, Howe TE, et al. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 2015; 2015(6): CD0061423. doi:10.1002/14651858.CD006142.pub3.
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effec-tiveness. J Pain 2003; 4(3): 109-121. doi: 10.1054/jpai.2003.434.
- Machado AF, Santana EF, Tacani PM, et al. The effects of transcutaneous electrical nerve stimulation on tissue repair: A literature review. Can J Plast Surg 2012; 20(4): 237-240. PMID: 24294017.
- Dailey DL, Rakel BA, Vance CGT, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013; 154(11): 2554-2562. doi: 10.1016/j.pain.2013.07.043. Epub 2013 Jul 27. PMID: 23900134.
- Dailey DL, Vance CGT, Rakel BA, et al. Transcutaneous Electrical Nerve Stimulation Reduces Movement-Evoked Pain and Fatigue: A Randomized, Controlled Trial. Arthritis Rheumatol 2020; 72(5): 824-836. doi: 10.1002/art.41170. Epub 2020 Mar 18. PMID: 31738014.
- Astokorki AHY, Mauger AR. Transcutaneous electrical nerve stimulation reduces exercise-induced perceived pain and improves endurance exercise performance. Eur J Appl Physiol 2017; 117(3): 483–492. doi: 10.1007/s00421-016-3532-6.
- Doré AL, Golightly YM, Mercer VS, et al. Lower limb osteoarthritis and the risk of falls in a community-based longitudinal study of adults with and without osteoarthritis. Arthritis Care Res 2015; 67(5): 633-639. doi: 10.1002/acr.22499.
- Mankovsky-Arnold T, Wideman TH, Lariviere C, et al. TENS attenuates repetition-induced summation of ac-tivity-related pain following experimentally induced muscle soreness. J Pain 2013; 14(11): 1416–1424. doi: 10.1016/j.jpain.2013.07.019.
- Butera KA, George SZ, Borsa PA, et al. Prolonged reduction in shoulder strength after transcutaneous electrical nerve stimulation treatment of exercise-induced acute muscle pain. Pain Pract 2018; 18(8): 954-968. doi: 10.1111/ papr.1269.
- Hibbert AW, Billaut F, Varley MC, et al. No Influence of Transcutaneous Electrical Nerve Stimulation on Exer-cise-Induced Pain and 5-Km Cycling Time-Trial Performance. Front Physiol 2017; 8: 26. doi: 10.3389/fphys.2017.00026.
- Menezes MA, Pereira TAB, Tavares LM, et al. Immediate effects of transcutaneous electrical nerve stimulation (TENS) administered during resistance exercise on pain intensity and physical performance of healthy subjects: a randomized clinical trial. Eur J Appl Physiol 2018; 118(9): 1941-1958. doi: 10.1007/s00421-018-3919-7.
- Johnson MI, Paley CA, Jones G, et al. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022; 12(2): e051073. doi: 10.1136/bmjopen-2021-051073.
- Boonstra AM, Schiphorst Preuper HR, Reneman MF, et al. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008; 31(2): 165-169. doi: 10.1097/MRR.0b013e3282fc0f93.
- Hong W-H, Lo S-F, Wu H-C, et al. Effects of compression garment on muscular efficacy, proprioception, and recovery after exercise-induced muscle fatigue onset for people who exercise regularly. Plos One 2022; 17(2): e0264569. doi: 10.1371/journal.pone.0264569.
- Leung AW, Chan CC, Lee AH, et al. Visual analogue scale correlates of musculoskeletal fatigue. Percept Mot Skills 2004; 99(1): 235-246. doi: 10.2466/pms.99.1.235-246.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. PMID: 7154893.
- Suzuki FS, Mazuchi FAS, Miranda MLJ, et al. What Is The Most Effective Protocol To Induce Fatigue In Knee Joint Muscles? A Systematic Review. Braz J Morphol Sci 2013; 30: 143-147.
- Rai SK, Yazdany J, Fortin PR, et al. Approaches for estimating minimal clinically important differences in sys-temic lupus erythematosus. Arthritis Res Ther 2015; 17(1): 143. doi: 10.1186/s13075-015-0658-6.
- Vance CG, Dailey DL, Rakel BA, et al. Using TENS for pain control: the state of the evidence. Pain Manag 2014; 4(3): 197-209. doi: 10.2217/pmt.14.13.
- Sluka KA, Bjordal JM, Marchand S, et al. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther 2013; 93(10): 1397-1402. doi: 10.2522/ptj.20120281.
- Denegar CR, Perrin DH. Effect of transcutaneous electrical nerve stimulation, cold, and a combination treatment on pain, decreased range of motion, and strength loss associated with delayed onset muscle soreness. J Athl Train 1992; 27(3): 200-206. PMID: 16558162; PMCID: PMC1317247.
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med 2001; 18: 205–207. doi: 10.1136/emj.18.3.205.
- Gibson W, Wand BM, Meads C, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of Cochrane Reviews. Cochrane Database Syst Rev 2019; 4(4): CD011890. doi: 10.1002/14651858.CD011890.pub3.
- Johnson MI, Jones G, Paley CA, et al. The clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain: a protocol for a meta-analysis of randomised controlled trials (RCTs). BMJ Open 2019; 9(10): e029999. doi: 10.1136/bmjopen-2019-029999.
- Lawson D, Degani AM, Lee K, et al. Use of transcutaneous electrical nerve stimulation along with functional tasks for immediate pain relief in individuals with knee osteoarthritis. Eur J Pain 2022; 26(3): 754-765. doi: 10.1002/ejp.1903. Epub 2022 Jan 6.
- Chabal C, Fishbain DA, Weaver M, et al. Long-term transcutaneous electrical nerve stimulation (TENS) use: impact on medication utilization and physical therapy costs. Clin J Pain 1998; 14: 66–73. doi: 10.1097/00002508-199803000-00010.
- Craig JA, Cunningham MB, Walsh DM, et al. Lack of effect of transcutaneous electrical nerve stimulation upon experimentally induced delayed onset muscle soreness in humans. Pain 1996; 67: 285–289. doi: 10.1016/0304-3959(96)03124-7.
- Kang DH, Jeon JK, Lee JH. Effects of low-frequency electrical stimulation on cumulative fatigue and muscle tone of the erector spinae. Journal of Physical Therapy Science 2015; 27(1): 105–108. doi: 10.1589/jpts.27.105.
- Denegar CR, Huff CB. High and low frequency TENS in the treatment of induced musculoskeletal pain: a comparison study. Athl Train J 1988; 23(3): 235-237.
